This video was published on 2022-03-16 17:49:55 GMT by @The-BMJ on Youtube.
The BMJ has total 40.5K subscribers on
Youtube and has a total of 303 video.This video has received 65.8K
Likes which are higher than the average likes that The BMJ gets . @The-BMJ receives an average views of 61.3K
per video on Youtube.This video has received 9.5K
comments which are higher than the average comments that The BMJ gets .
Overall the views for this video was lower than the average for the profile.



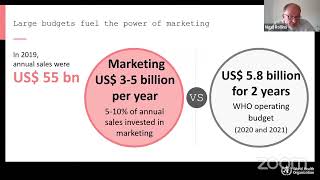

















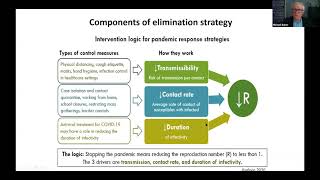



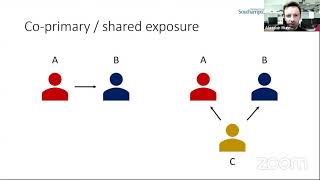





























The BMJ's video: Covid-19: Researcher blows the whistle on data integrity issues in Pfizer s vaccine trial
65.8K
9.5K